A new method for production of titanium dioxide pigment
Abstract
Titanium dioxide (TiO2) has been widely used as pigment in paints, paper and cosmetic products, as well as high-tech applications such as solar cells, semiconductors, biomedical devices and air purification. TiO2 pigment is primarily produced by a high temperature chloride process, which forms CO2 as a reaction byproduct. A novel hydrometallurgical process for making TiO2 pigment without direct CO2 emission is investigated. The novel process involves alkaline roasting of titania slag, with subsequent washing, leaching, solvent extraction, hydrolysis, and calcination stages, resulting in high-purity anatase or rutile pigments. Experimental validation for each of the processing steps is demonstrated. Pigment whiteness is critically sensitive to trace amounts of discoloring impurities such as iron. The use of solvent extraction proved to be highly effective in reducing the concentration of discoloring impurities in the final pigment to commercially acceptable levels.
Highlights
► We report a new process for producing TiO2 pigment without direct CO2 emissions.
► The process involves slag roasting, leaching, SX, hydrolysis, and calcination.
► Experimental validation of the process chemistry is demonstrated.
► TiO2 pigment with impurity levels comparable to commercial pigment produced
Introduction
Titanium dioxide (TiO2) is one of the most commonly used minerals in the chemical manufacturing industry. It has been commercially processed since the early 1900s, and has a wide variety of applications. Pigment is used in paints, plastics and paper (Gambogi, 2010), sunscreen (Yuan et al., 2005), cosmetics and even as a food additive (Kuznesof, 2006). TiO2 has also been used in photovoltaic cells (Gratzel, 2001), biomedical devices (Yang et al., 2009), and in air purification (Hassan, 2009). The rutile form of TiO2 has a refractive index of 2.7, which imparts a high level of opacity (and hence whiteness) to whatever material it is added (Patton, 1973). It should be noted that even diminutive concentrations of other transition metals such as iron can have a significant impact on the whitening ability of the pigment.
The two main commercial processes for producing titanium dioxide pigment are sulfate process (Barksdale, 1966) and chloride process (Winkler, 2003). The main feed stocks for these processes are high-TiO2 slag and/or synthetic rutile produced from ilmenite ore. The most common method for producing titania slag is smelting process which reduces iron oxides to liquid iron in large electric arc furnaces (Sahu et al., 2006).The main product of smelting is titania slag containing approximately 75 to 85% TiO2. The co-product of smelting is pig iron, which can be used as raw material for the steel industry. Titania slag can be used to produce synthetic rutile, which is a further upgraded raw material with a TiO2 content of 92–96%. However, the major world production of synthetic rutile is by a combination of partial reduction and acid leaching steps, such as in the Becher or Benilite processes (Balderson and MacDonald, 1999). The energy consumed in producing either slag or synthetic rutile is roughly equivalent (35.5 and 35.0 MJ/ton TiO2, respectively) (Reck and Richards, 1997). However, the upgrading of ilmenite to synthetic rutile produces acidic effluents as well as a considerable amount of solid waste (2 tons/ton TiO2) (European Commission, 2007). For this reason, titania slag could be considered a more economical and environmentally friendly upgraded feed material.
Titania slag or raw ilmenite is used as the feed material for the sulfate process, which was developed nearly 100 years ago. The feed material is digested with concentrated sulfuric acid to produce titanium sulfate followed by the bulk removal of iron through crystallization and filtration steps. The titanium sulfate is hydrolyzed at boiling temperatures and a hydrous titanium oxide compound is precipitated and removed from solution. This compound is calcined at 650–1000 °C to form either anatase or rutile-type TiO2. The major advantages of this process include low capital costs and flexibility in feed material. The major disadvantages include an abundant generation of acidic and solid waste, a large consumption of energy, and a variable quality of product due to batch (as oppose to continuous) processing. Despite these disadvantages, this process still accounts for 40% of the total TiO2 pigment produced world-wide (Xue et al., 2009).
The chloride process was first developed in the late 1950s by DuPont. The chloride process involves reacting rutile (natural or synthetic) or titania with petroleum coke and chlorine gas at high temperatures, forming a titanium tetrachloride (TiCl4) vapor. The vapor is distilled and then oxidized at 1300–1800 °C with oxygen and AlCl3. The key reactions for this process are shown below.TiO2 + C + 2Cl2 → TiCl4 + CO22FeTiO3 + 3C + 7Cl2 → 2TiCl4 + 2FeCl3 + 3CO2TiCl4 + O2 → TiO2 + 2Cl2
The resulting product of these reactions is a highly purified TiO2 pigment. While the process does not produce the same magnitude of wastes as the sulfate method, it still has several environmental issues of its own. From the stoichiometry of Reaction (1), we can see that 1 mol of CO2 is directly generated for every mol of TiO2 produced, which equates to 550 kg CO2 per ton TiO2 produced. The world production capacity of titanium dioxide pigment by the chloride process is an estimated 3.4 million metric tons per year (Gambogi, 2010), resulting in approximately 1.9 million tons per year of CO2 emitted from this reaction alone.
Several hydrometallurgical processes for producing pigment grade TiO2 have been in development in the last decade as alternatives to the chloride process. Most of these processes involve acid leaching of ilmenite or upgraded feeds. Roche et al. (2004) have developed an improved sulfate process that uses sulfuric acid streams more efficiently by reusing the raffinate from a solvent extraction step as the lixiviant for the initial leaching step, resulting in a reduction in acidic effluents and solid waste neutralization products. Using this process they are able to produce a pigment with 99% purity and total Fe content of 0.07% (700 ppm) (Roche et al., 2004).
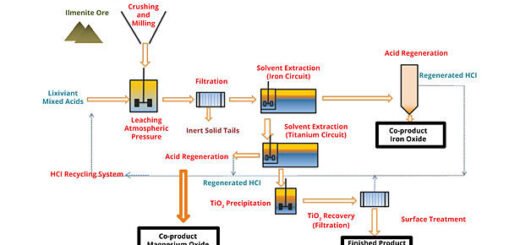
A direct leaching method of ilmenite using chloride media has been developed by Lakshmanan et al (2010). The process involves leaching the ore between 65 and 80 °C in 20% HCl and 150–300 g/L MgCl2. Iron impurities are removed from the leach solution by solvent extraction using TBP and recovered as iron oxides through pyrohydrolysis. This process produced a pigment with a composition of > 99.0% TiO2 and < 0.01 % Fe (100 ppm) (Lakshmanan et al., 2010).
The Altair process (Verhulst et al., 2002) uses concentrated HCl (> 360 g/L) to directly leach ilmenite. The solution is cooled to crystallize FeCl2 for bulk iron removal and fed to a two stage solvent extraction procedure that uses a phosphine oxide to extract titanium and ferrous ions in the first stage and an amine extractant to remove ferric ions in the second. The purified titanium solution is hydrolyzed in a spray dryer and calcined to produce TiO2 pigment particles. Pigments with iron concentrations of 6 ppm have been achieved. This process also uses pyrohydrolysis to regenerate acid. While these processes reduce the generation of acidic wastes, the pyrohydrolysis procedure can be quite energy intensive, and the use of concentrated HCl solutions requires high capital costs for process equipment.
In summary, the existing commercial processes for making TiO2 pigment consume large amounts of energy and/or generate large amounts of carbon emissions and solid waste. Another challenge includes producing a pigment with sufficient purity. For example, although each pigment application has a unique set of purity specifications, most commercial pigments require Fe levels of less than 50 ppm (Duyvesteyn et al., 2003), and some manufacturers produce pigment with less than 10 ppm.
Therefore, it is imperative that any novel process of pigment manufacture be able to realize certain advantages over current methods, but also be able to meet commercial pigment purity standards. A new method has been developed recently that relies on a combination of alkaline roasting of Ti-slag, leaching in HCl acid, and one-stage solvent extraction (Fang et al., 2011). The key objectives of the new process are to produce high-quality TiO2 pigment while reducing energy consumption and eliminating the direct CO2 emissions of the chloride process. This paper describes the specific methods that are used in this new process and reports the results of laboratory experiments using the new process. A forthcoming analysis by the authors compares the estimated energy consumption and carbon emissions of the new process with those of the chloride and sulfate processes.